Research Article - International Journal of Pharmaceutical, Chemical and Biological Sciences ( 2021) Volume 11, Issue 2
SYNTHESIS OF 2-(NAPHTHO[2,1-B]FURAN-2-YL)-5-PHENYL-1,3,4-OXADIAZOLE DERIVATIVES AND THEIR ANTIMICROBIAL ACTIVITIES
MN Kumaraswamy1*, D Ramesh1, BK Nagaraj2 and TS Rashmi32Department of Microbiology, Sir. M. V. Government Science College, India
3Department of Biotechnology, Sir. M. V. Government Science College, India
MN Kumaraswamy, Department of Chemistry, Sir. M. V. Government Science College, India,
Published: 20-Oct-2021
Abstract
The reaction of ethyl naphtho[2,1-b]furo-2-carboxylate (2) with hydrazine hydrate in presence of catalytic amount conc. HCl in ethanol at reflux temperature afforded naphtho[2,1-b]furan-2- carbohydrazide (3) in good yield. The reaction of naphtho[2,1-b]furan-2-carbohydrazide with aromatic aldehydes (4a-f) in ethanol in the presence of acid catalyst produces N1-[substitutedphenylmethylidene] naphtho[2,1-b]furan-2-carbohydrazide (5a-f). These N1-[substituted-phenyl methylidene]naphtho[2,1-b]furan-2-carbohydrazide 5a-f on treatment with 1.2 equivalence of iodine and potassium carbonate in DMSO produces 2-(naphtho[2,1-b]furan-2-yl)-5-(substituted) phenyl 1,3,4-oxidiazole derivatives (6a-f). The structures of newly synthesized compounds have been established by elemental analysis and spectral studies. These compounds have been screened for antimicrobial activities.
Introduction
The chemistry of the compounds containing the condensed oxadiaoles play significant role in pharmaceutical industry. Among various oxadiazoles, 1,3,4-oxadiazoles and its derivatives attracted attention due to their wide spectrum of biological and pharmacological activities such as antimicrobial, antifungal [1-4], anticonvulsant activity [5,6], anti-inflammatory, analgesic [7], antitumor [8,9] and other biological activities [10,11]. The derivatives of 1,3,4-oxadiazoles act as HIV reverse transcriptase inhibitors [12]. Several derivatives of naphtho[2,1-b]furan synthesized in our laboratory have been reported to possess many biological and pharmacological activities such as antimicrobial, analgesic, anti-inflammatory, diuretic, anthelmintic, antipyretic etc [13-15].
Survey of literature revealed that, similar type of work involving 1,3,4-oxadiazole and naphtho[ 2,1-b]furan either in condensed form or in coupled form has not been reported. Hence it is thought of interest to synthesize 2-(naphtho[ 2,1-b]furan-2-yl)-5-phenyl-1,3,4-oxadiazole derivatives and evaluate them for antibacterial and antifungal activities.
Experimental Methods and Characterization
Step 1: Synthesis of 2-hydroxy-1-naphthaldehyde (1)
2-Naphthol (0.04 mol) was dissolved in 15 ml ethanol to this NaOH (0.25 mol) of in 21 ml water was added and kept stirring. To this 4.2 ml of chloroform was added drop wise, after the completion of addition the reaction mixture kept for stirring for about 2 hours. The reaction mixture was poured to ice cold water and neutralized with dilute HCl solid separates filtered and dried. The product obtained was recrystallised from ethanol.
Step 2: Synthesis of ethyl naphtho[2,1-b]furan- 2-carboxylate (2)
To a solution of 2-hydroxy-1-naphthaldehyde (1) (0.03 mol) in dry N, N-dimethylformamide (25 ml), ethyl chloro acetate (0.03 mol) and anhydrous potassium carbonate (0.9 mol) were added and the reaction mixture was refluxed on water bath for 24 hours. The reaction mixture was filtered and potassium carbonate was washed with DMF.
The filtrate was concentrated by distillation then poured into ice cold water, to obtain the product as solid which was collected by filtration, dried and recrystallised from ethanol (2).
The IR spectrum of (2) exhibited the absorption band at 1732 cm-1 due to C=O of ester group. In 1H NMR spectrum (CDCl3) triplet at d 1.5 due to -CH3 protons, a quartet at δ 4.5 due to –CH2 protons and a multiplet at δ 7.6-8.2 integrating for 7 aromatic protons.
Step 3: Synthesis of naphtho[2,1-b]furan-2-carbohydrazide (3)
Ethyl naphtho[2,1-b]furo-2-carboxylate (2) (0.01 mol), catalytic amount of conc. hydrochloric acid and hydrazine hydrate (0.02 mol) were refluxed in absolute ethanol (25 ml) for 2 hrs on water bath. Then the reaction mixture was cooled to room temperature, the solid thus obtained was filtered and dried. The product obtained was recrystallised from ethanol (3).
The structure of (3) was well confirmed by elemental analysis and spectral studies. The IR spectrum of (3) exhibited broad absorption band at 3304-2969 cm-1 due to NH2 and a sharp absorption band at 1657 cm-1 due to C=O group. 1H NMR spectrum of (3) shows a broad singlet at d 4.6, 1H, NH, (D2O exchangeable), multiplet d 7.4-8.6, for 7 aromatic protons and a singlet at d 9.8 for 2 NH2 protons.
Step 4: Synthesis of N1-[substituted-phenylmethylidene] naphtho[2,1-b]furan-2-carbohydrazide (5a-f).
The reaction of naphtho[2,1-b]furan-2-carbohydrazide (0.452 gm 0.002 mol) with anisaldehyde (0.27 gm, 0.002mol) in ethanol (10 ml) at reflux temperature in presence of Conc. HCl undergo condensation and produces N’-[(4methoxyphenyl) methylidene]naphtho[2,1-b]furan-2-carbohydrazide (5d).
The structure of (5d) was well confirmed by spectral studies. The IR spectrum of (5d) exhibited broad absorption band at 3300-2970 cm-1 due to NH, a sharp absorption band at 1652 cm-1 due to C=O group and band at 1585 cm-1 due to C=N. In 1H NMR spectrum (CDCl3) singlet at δ 3.9 integrating for 3-OCH3 protons, singlet at d 4.4, for 1 NH proton and a multiplet at δ 7.6-8.6 integrating for 11 aromatic protons. The same method employed for the synthesis of (5a-c) and (5e-f).
Step 5: Synthesis of 2-(naphtho[2,1-b]furan- 2-yl)-5-(substituted) phenyl 1,3,4-oxidiazole (6a-f)
The reaction of N1-[substituted-phenylmethylidene] naphtho[2,1-b]furan-2-carbohydrazide (0.344 gm 0.001 mol) with iodine (1.2 equivalent 0.30 gm) and potassium carbonate (0.69 gm 0.005 mol) in DMSO 10 ml refluxed for 8 hours produces 2-(4-methoxyphenyl)-5-(naphtho[2,1-b]furan-2- yl)-1,3,4-oxadiazole (6d). The same method was followed for the synthesis of compounds (6a-c) and (6e-f) from (5a-c) and (5e-f) (Figure 1).
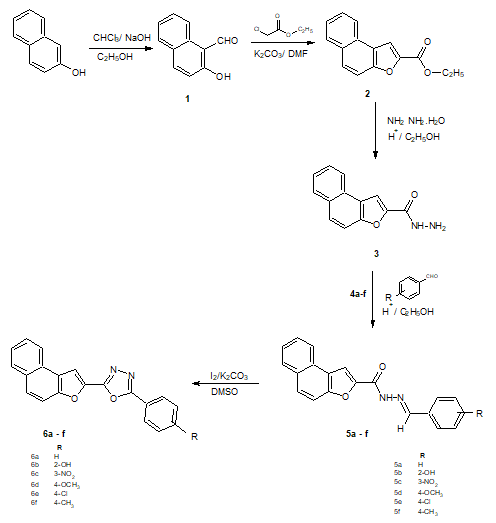
Figure 1: Synthetic route-1
The structure of (6d) was well confirmed by elemental analysis and spectral studies. 1H NMR (DMSO-d6) spectrum of (6d) shows a singlet at d 3.8, 3H, OCH3 and a multiplet d 7.6-8.6, for 11 aromatic protons. The physical data of newly synthesized compounds were reported in (Table 1). The compounds encompassing naphthofuran and oxadiazole are known to exhibit wide spectrum of biological and pharmacological activities. Hence, it was intrigued to evaluate newly synthesized compounds for antimicrobial activities by adopting literature procedure [13,14]. The results were reported in (Table 2).
| Comp. | R | M.p. 0C | Yield (%) | Mol. formula | Clad (Found) % | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| 1 | ----- | 80 | 65 | C12H10O2 | 77.4 | 5.41 | ---- |
| 77.28 | 5.29 | ||||||
| 2 | ---- | 100 | 63 | C15H12O3 | 74.99 | 5.03 | 19.98 |
| 74.86 | 4.89 | 19.81 | |||||
| 3 | ---- | 270 | 67 | C13H10N2O2 | 69.02 | 4.46 | 12.38 |
| 68.85 | 4.32 | 12.25 | |||||
| 5a | H | 109 | 71 | C20H14 N2O2 | 76.42 | 4.49 | 8.91 |
| 76372 | 4.37 | 8.82 | |||||
| 5b | 2-OH | 134 | 67 | C20H14 N2O3 | 72.72 | 4.27 | 8.28 |
| 72.61 | 4.18 | 8.16 | |||||
| 5c | 3-NO2 | 141 | 68 | C20H13 N3O4 | 66.85 | 3.65 | 11.9 |
| 66.76 | 3.54 | 11.81 | |||||
| 5d | 4-OCH3 | 168 | 69 | C21H16 N2O3 | 73.24 | 4.68 | 8.13 |
| 73.13 | 4.56 | 8.01 | |||||
| 5e | 4-Cl | 153 | 71 | C20H13 ClN2O2 | 68.87 | 3.76 | 8.03 |
| 68.77 | 3.65 | 7.91 | |||||
| 5f | 4-CH3 | 127 | 71 | C21H16 N2O2 | 76.81 | 4.91 | 8.53 |
| 76.7 | 4.82 | 8.42 | |||||
| 6a | H | 143 | 68 | C20H12 N2O2 | 76.91 | 3.87 | 8.97 |
| 76.8 | 3.76 | 8.86 | |||||
| 6b | 2-OH | 149 | 66 | C20H12 N2O3 | 73.16 | 3.68 | 8.53 |
| 73.07 | 3.57 | 8.41 | |||||
| 6c | 3-NO2 | 165 | 67 | C20H11 N3O4 | 67.23 | 3.1 | 11.76 |
| 67.11 | 3 | 11.64 | |||||
| 6d | 4-OCH3 | 189 | 69 | C21H14 N2O3 | 73.68 | 4.12 | 8.18 |
| 73.53 | 4.02 | 8.05 | |||||
| 6e | 4-Cl | 170 | 65 | C20H11 ClN2O2 | 69.27 | 3.20 | 8.08 |
| 69.18 | 3.11 | 7.99 | |||||
| 6f | 4-CH3 | 156 | 65 | C21H14 N2O2 | 77.29 | 4.32 | 8.58 |
| 77.2 | 4.21 | 8.49 | |||||
Table 1: Physical data of newly synthesized compounds.
| Comp. | Antibacterial activity | Antifungal activity | ||
|---|---|---|---|---|
| P. aerugenosa | S. aureus | A. niger | C. Lunata | |
| 6a | 20 | 20 | 19 | 19 |
| 6b | 21 | 19 | 20 | 19 |
| 6c | 19 | 18 | 19 | 19 |
| 6d | 19 | 18 | 19 | 18 |
| 6e | 18 | 18 | 18 | 18 |
| 6f | 17 | 17 | 18 | 17 |
| Standard | 24 | 24 | 24 | 26 |
| DMF | NIL | NIL | NIL | NIL |
Table 2: The results of antimicrobial activities.
Results and Discussion
With the literature survey, it was found that the derivatives of the 1,3,4-oxadiazole show action as the HIV reverse transcriptase inhibitors and several naphtho[2,1-b]furan derivatives show the analgesic, anti-inflammatory, antipyretic, antibacterial as well as the antifungal activities and therefore the derivatives of 2-(naphtho[2,1-b]furan-2- yl)-5-phenyl-1,3,4-oxadiazole were synthesized and evaluated for their mentioned properties. By reacting ethyl naphtho[2,1-b]furo-2-carboxylate (2) with hydrazine hydrate in the presence of concentrated HCl in an ethanol solution at the an optimum temperature, naphtho[2,1-b]furan-2-carbohydrazide (3) was formed. Further reactions of the naphtho[2,1-b]furan-2-carbohydrazide with aromatic aldehydes in the presence of acid catalyst in ethanol, N1-[substituted-phenylmethylidene] naphtho[2,1-b]furan-2-carbohydrazide (5a-f) was formed and these N1-[substituted-phenyl methylidene]naphtho[2,1-b]furan-2-carbohydrazide 5a-f when treated in the presence of DMSO with iodine and potassium carbonate, it produces 2-(naphtho[2,1-b]furan-2-yl)-5-(substituted) phenyl 1,3,4-oxidiazole derivatives (6a-f).
The compounds were synthesized and their several biological as well as the pharmacological activities were evaluated by applying or performing the elemental or structural analysis and the spectral studies. Firstly, the 2-hydroxy-1-naphthaldehyde was synthesized and then ethyl naphtho[2,1-b] furan-2-carboxylate (2) was synthesized, where by performing one of the spectral analysis i.e., IR spectroscopy, which has shown its absorption band at 1732 cm-1 due to the presence of the C=O of ester group. In 1H NMR spectrum (CDCl3) triplet at d 1.5 due to -CH3 protons, a quartet at δ 4.5 due to –CH2 protons and a multiplet at δ 7.6-8.2 integrating for 7 aromatic protons. Later on, the naphtho[2,1-b]furan-2-carbohydrazide (3) was synthesized and its IR spectrum of (3) exhibited broad absorption band at 3304-2969 cm-1 due to the presence of NH2 and a sharp absorption band at 1657 cm-1 due to the presence of C=O group. While coming to the NMR studies, 1H NMR spectrum of (3) has shown a broad singlet at d 4.6, 1H, NH, (D2O exchangeable), multiplet d 7.4-8.6, for 7 aromatic protons and a singlet at d 9.8 for 2 NH2 protons.
The another compound that has been synthesized and analyzed was N1-[substituted-phenylmethylidene] naphtho[2,1-b]furan-2-carbohydrazide. After synthesizing the compound, the IR spctra of (5d) have been exhibited with the broad band as well as the sharp absorption band. The broad band was seen at 3300-2970 cm-1 due to the presence of NH. The sharp absorption band was seen at the 1652 cm-1 due to the presence of C=O group and another band at 1585 cm-1 due to the presence of C=N. In NMR studies, 1HNMR spectrum has shown a singlet at δ 3.9 integrating for 3 -OCH3 protons, another singlet at d 4.4, for one NH proton and a multiplet at δ 7.6-8.6 integrating for 11 aromatic protons. The same method employed for the synthesis of (5a-c) and (5e-f). After synthesizing 2-(naphtho[2,1-b]furan-2-yl)-5-(substituted) phenyl 1,3,4-oxidiazole (6a-f), the NMR studies i.e., 1H NMR (DMSO-d6) spectrum of (6d) shown a singlet at d 3.8, 3H, OCH3 and a multiplet d 7.6-8.6, for 11 aromatic protons.
The various compounds were synthesized and evaluated through the elemental as well as the spectral analysis. From the data obtained after analyzing, the newly synthesized compounds that are exhibiting the naphthofuran and oxadiazole are found to be shown with the wide spectrum of biological and pharmacological activities. Therefore, the newly synthesized compounds exhibit the antimicrobial activities so as the literature survey.
Conclusion
The newly synthesized compounds were evaluated for antimicrobial activity. All the compounds were displayed significant antibacterial activity against both the organisms. It is observed that electron withdrawing groups resulted in enhancement of activity.
Acknowledgements
The authors are thankful to The Principal, Sir. M. V. Government Science College, Bhadravathi, for the encouraging support and for providing the laboratory facilities. The authors are thankful to the Head, Sophisticated Instruments Facility, IISc, Bangalore for Spectral data.
References
- Kadi AA, El-Brollosy NR, Al-Deeb OA, Habib EE, Ibrahim TM and et al. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazoles. Eur J Med Chem 2007; 42:235-242.
- Sengupta P, Mal M, Mandal S, Singh J and Maity TK. Evaluation of antibacterial and antifungal activity of some 1,3,4-oxadiazoles. Iranian J Pharma and Therap 2008; 7:165-167.
- Bhardwaj N, Saraf SK, Sharma P and Kumar P. Synthesis, evaluation and characterization of some 1, 3, 4-oxadiazoles as antimicrobial agents. E-J Chem 2009; 8:240-244.
- Omar FA, Mahfouz NM and Rahman MA. Design, synthesis and anti-inflammatory activity of some 1,3,4-oxadiazole derivatives. Eur J Med Chem 1996; 31:819-825.
- Kashaw SK, Gupta V, Kashaw V, Mishra P, Stables JP and et al. Anticonvulsant and sedative-hypnotic activity of some novel 3-[5-(4-substituted) phenyl-1,3,4-oxadiazole-2yl]-2- styrylquinazoline-4(3H)-ones. Med Chem Res 2010, 19:250-261.
- Bhat MA, Al-Omar MA and Siddiqui N. Synthesis, anticonvulsant and neurotoxicity of some novel 1,3,4-oxadiazole derivatives of phthalimide. Der Pharma Chemica 2010; 2:1-10.
- Husain A, Ahmad A, Alam MM, Ajmal M and Ahuja P. Fenbufen based 3-[5-(substituted aryl)-1,3,4-oxadiazol-2-yl)]-1-(biphenyl-4-yl)propan-1-ones as safer anti-inflammatory and analgesic agents. Eur J Med Chem 2009; 44:3798-3804.
- Savariz FC, Formagio ASN, Barbosa VA, Foglio MA, Carvalho JE and et al. Synthesis, antitumor and antimicrobial activity of novel 1-substituted phenyl-3-[3-alkylamino(methyl)-2-thioxo-1,3,4-oxadiazol-5-yl]-b-carboline derivatives. J Braz Chem Soc 2010; 21:288-298.
- Bondock S, Adel S, Etman HA and Badria FA. Synthesis and antitumor evaluation of some new 1,3,4-oxadiazole-based heterocycles. Eur J Med Chem 2012; 48:192-199.
- Puthiyapurayil P, Poojary B, Chikkanna C and Buridipad SK. Design, synthesis and biological evaluation of a novel series of 1,3,4-oxadiazole bearing N-methyl-4-(trifluoromethyl) phenyl pyrazole moiety as cytotoxic agents. Eur J Med Chem 2012; 53:203-210.
- Chandrakantha B, Shetty P, Nambiyar V, Isloor N and Isloor AM. Synthesis, characterization and biological activity of some new 1,3,4-oxadiazole bearing 2-flouro-4-methoxi phenyl moiety. Eur J Med Chem 2010;45:1206-1210.
- El-Emam AA, Al-Deeb OA, Al-Omar M and Lehmann J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg Med Chem 2004;12:5107-5113.
- Kumaraswamy MN, Vaidya VP, Chandrasekhar C, Prathima Mathias DA, Shivakumar H and et al. Synthesis of Novel 5,8-Dihydro[1,2,4]Triazolo[3,4-B][1,3,4]thiadiaze pines derivatives involving naphtho[2,1-b]furan and evaluation of their possible pharmacological activities. International Journal Of Pharmaceutical And Chemical Sciences 2016;5:245-251.
- Kumaraswamy MN, Prathima Mathias DA, Chandrashekhar C, Shivakumar H, Mahadevan KM and et al. Novel approach towards the synthesis of 2-(1-naphtho[2,1-b]furan-2-yl carbonyl)-3,5-disubstituted-2,3-dihydro-1H-pyrazoles: As a new class of antimicrobial and pharmacological agents. Indian J Pharma Sci 2008;70:715-720.
- Ramesh D, Vaidya VP, Kumaraswamy MN and Chandrashekhar C. Synthesis of 2-(8-bromonaphtho[2,1-b]furan-2-yl)-5-aryl-1,3,4- Oxadiazoles as Potential Antimicrobial agents. International Journal of Pharmaceutical, Chemical and Biological Sciences 2014; 4(2): 298-306.

